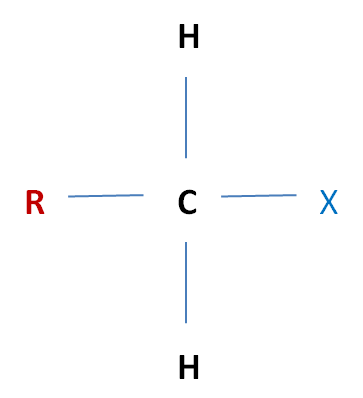
Halogenoalkanes are compounds made up of alkanes and halogens. The General formula being CnH2n+1X (X being a halogen atom: one of F, Cl, Br or I)
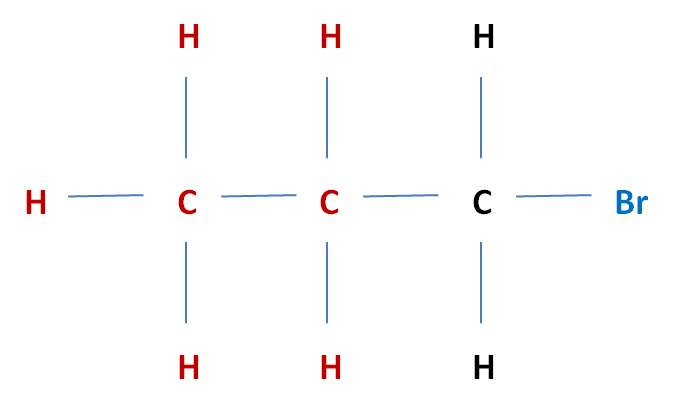
Classifying Halogenolkanes Much like alcohols, halogenoalkanes can be classified according to their structures, as either primary, secondary or tertiary. Primary (1°) Halogenoalkane The carbon which is carrying the halogen atom, is attatched to one other alkyl group
Eg. 1-Bromopropane
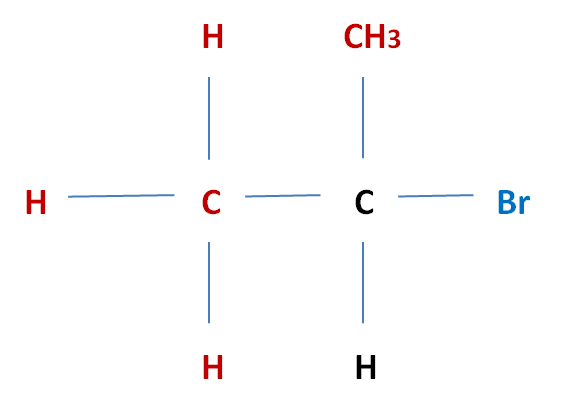
| Secondary (2°) Halogenoalkane
Eg. 2-Bromo-propanen (even though its a branch, you still need to use the laws of naming alkanes) Halogen atom is attatched to a carbon atom which is then attatchad to two alkyl groups
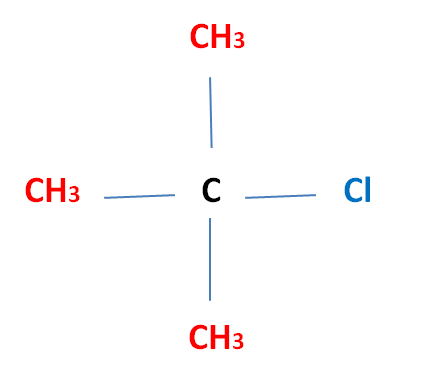
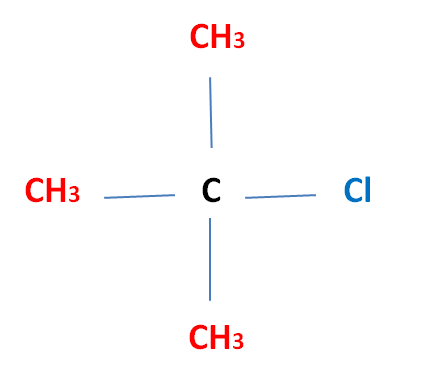
Tertiary (3°) Halogenoalkane The halogen atom is attatched to a carbon atom which is bonded to three other alkyl groups Eg. 2-Methyl-2-ChloroPropane
|